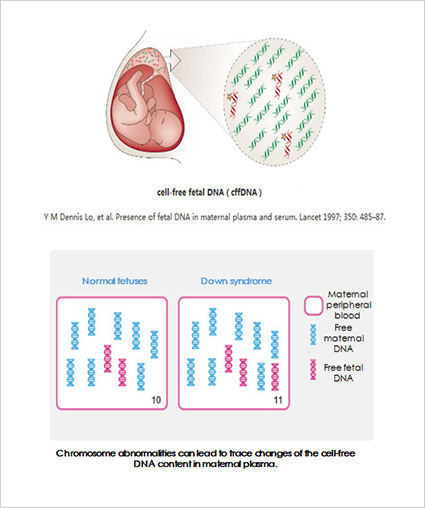
Using the international leading high-throughput sequencing platform and bioinformatics analysis, NIFTY is able to analyse the risk ratio of trisomy 21 (Down syndrome), trisomy 18 (Edward syndrome), trisomy 13 (Patau syndrome) , and other chromosomal aneuploidy by extracting free DNA from maternal blood.


Conduct pre-test counseling with the pregnant woman

Sign informed consent, pay and receive blood bag

Carry out 10ml venous blood draw from the pregnant woman

DNA sequencing will be tested at laboratory within 8 hours after sampling

Test results can be received in 10 working days later

Genetic testing for single gene disorders is used to test kinds of common single-gene diseases by 5mL peripheral blood or 2mL saliva by adopting target sequence capture combined with high-throughput sequencing technology. Therefore, it can quickly and accurately help couples of childbearing age to understand pathogenic mutations carried by their own, and the risk ratio of having children with disease that will ensure optimal plans of pregnancy and, ultimately, the birth of a healthy newborn
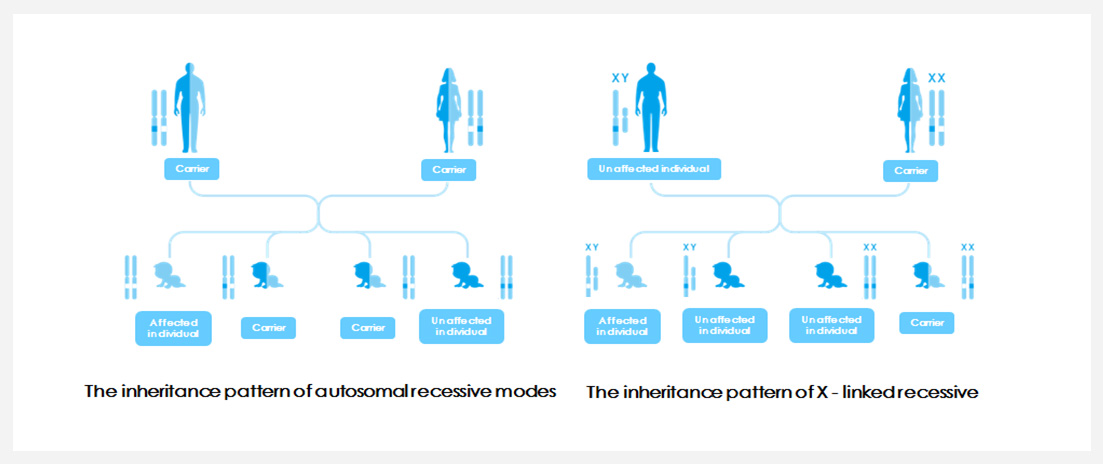
Because of the normal phenotype of implicit genetic disease carriers, routine prenatal examination often can not be detected, pathogenic mutations from generation to generation, until the parents give birth to children with disease are found. In the pre-pregnancy or early pregnancy to carry out screening, the birth defects in advance prevention ,and thus can fundamentally block the inheritance of single gene disease.
More and more medical studies have shown that the vast majority of human diseases are closely linked with genes. Because the genetic differences, there is a big difference in the probability that individual suffer from certain diseases in the life, some people are high and some people are low. However, by detecting susceptibility genes related precisely, the probability of occurrence of disease can be assessed in our project
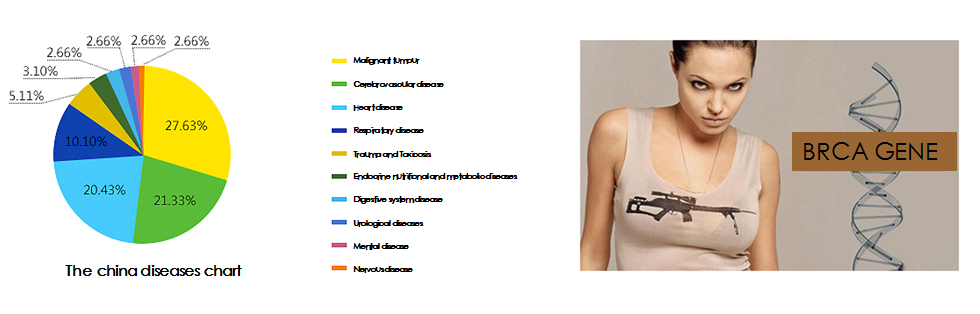
Among all the diseases, chronic diseases are one of the major threats to the health of our country. According to statistics, there are 270 million chronic diseases in China in 2008, and this number is growing at an annual rate of 16 million cases. The number of deaths from chronic diseases is as high as 6.43 million, and among them, malignancies, cerebrovascular disease, and heart disease account for the major part.
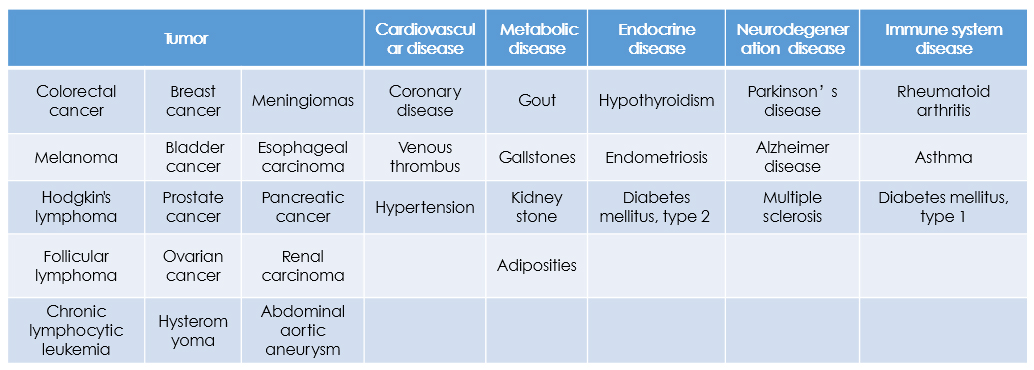
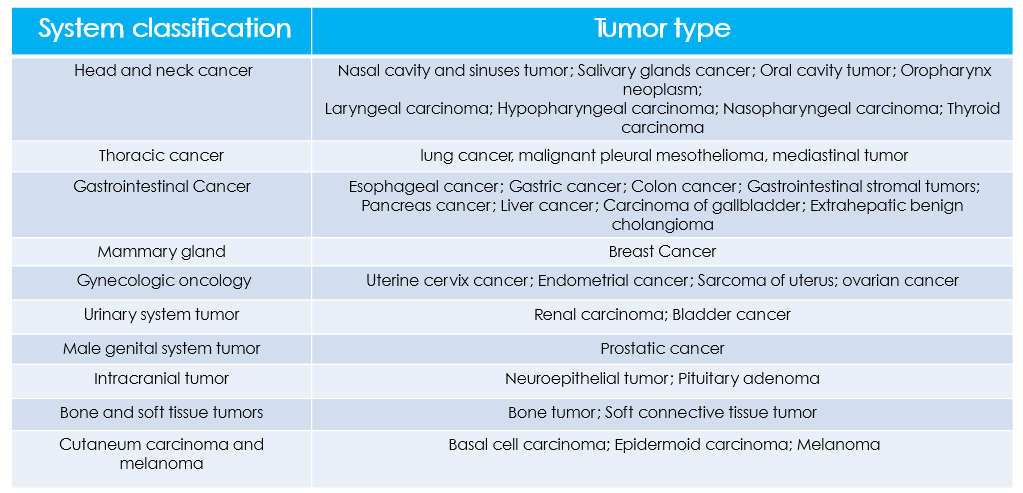
By adopting the new generation of high-throughput sequencing platform, the international leading of circulating single-molecule amplification and resequencing technology (cSMART), and target sequence capture combined with high-throughput sequencing technology, tumor whole-genome testing (also known as molecular diagnosis of tumor) can detect 343 kinds of genes associated with solid tumors and 414 blood system-related tumor genes at one time.
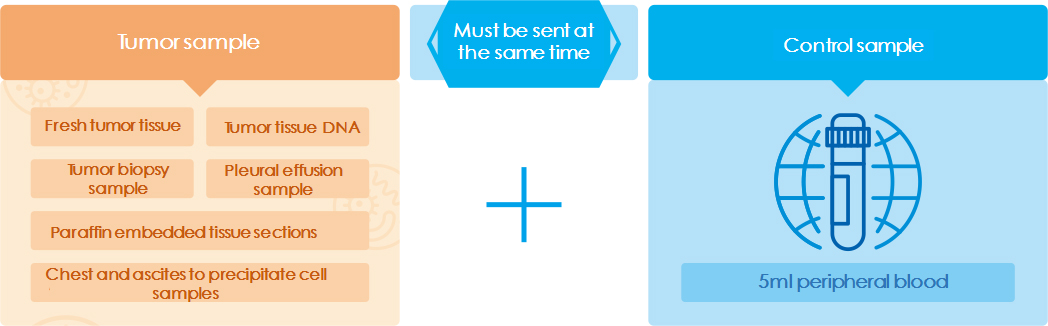
For people: Cancer patients, family history of hereditary tumors Sampling: Tumor tissue, paraffin section, or 10ml peripheral blood Accurate: Accurate detections of SNV, Indel, Fusion, and other mutations Comprehensive: One sampling, simultaneous testing multiple genes, multiple loci, and multiple mutations Fast: The written report of results can be received in 10 -15 working days later

Conduct pre-test counseling with the Patient

Sign informed consent

Sampling (tumor tissue, paraffin section or 10ml peripheral blood )

Sample will be tested at laboratory within 8 hours after sampling

The written report of results can be received in 14 working days later
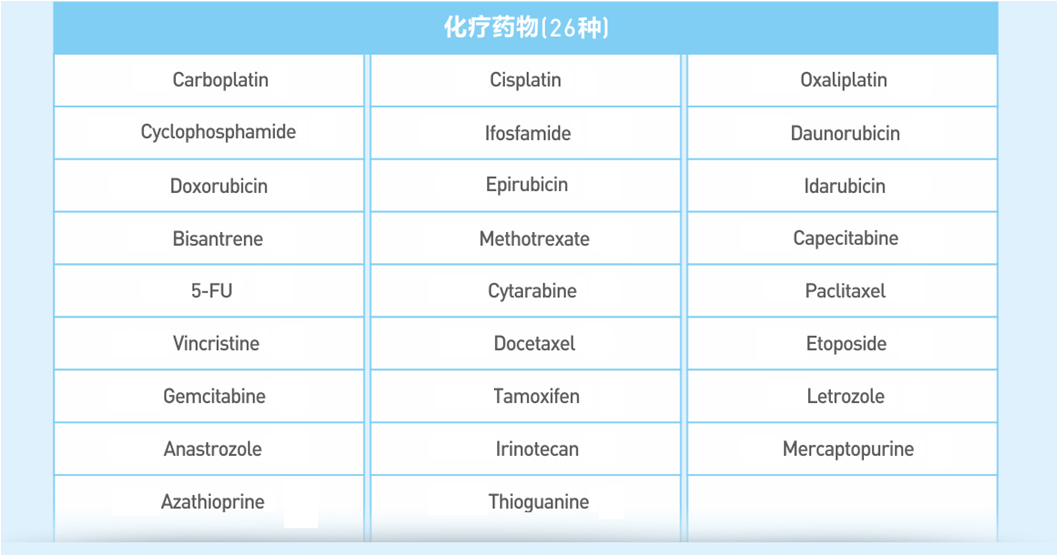
Genetic testing for tumors individualized medication, specifically for cancer patients, is a one-time accurate testing of the exogenous sub-region of 559 kinds of tumor-related genes by cancer tissue samples or peripheral blood samples with using a new generation of high-throughput sequencing platform. Hence, it can interpret the relationship between 75 kinds of tumor drugs and genes comprehensively and accurately, and learn more about tumor patients with specific genetic variation, thus to provide patients with more options for drug programs and, ultimately, to help doctors develop a better treatment program.